When an unstable or radioactive nucleus disintegrates spontaneously,
certain kinds of particles and/or high-energy photons are released. These
particles and photons are collectively called “rays.” Three kinds of rays are
produced by naturally occurring radioactivity:
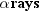
,
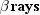
, and
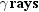
. They are named according to the
first three letters of the Greek alphabet, alpha (

), beta (
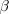
), and gamma (

), to indicate the extent of their
ability to penetrate matter.

rays are the least penetrating, being blocked by a
thin
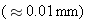
sheet of
lead, whereas
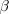
rays penetrate lead to a much greater distance
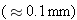
.

rays are the most penetrating and
can pass through an appreciable thickness
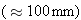
of lead.
 CONCEPTS AT A
GLANCE The nuclear disintegration process that produces
CONCEPTS AT A
GLANCE The nuclear disintegration process that produces  ,
, 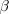 , and
, and  rays must obey the laws of physics
that we have studied in previous chapters. As the Concepts-at-a-Glance chart in
Figure 31-6 reminds us, these laws are called conservation laws because each of
them deals with a property (such as mass/energy, electric charge, linear
momentum, and angular momentum) that is conserved or does not change during a
process. To the first four conservation laws in Figure 31-6, we now add a fifth,
the conservation of nucleon number. In all radioactive decay processes it has
been observed that the number of nucleons (protons plus neutrons) present before
the decay is equal to the number of nucleons after the decay. Therefore, the
number of nucleons is conserved during a nuclear disintegration. As applied to
the disintegration of a nucleus, the conservation laws require that the energy,
electric charge, linear momentum, angular momentum, and nucleon number that a
nucleus possesses must remain unchanged when it disintegrates into nuclear
fragments and accompanying
rays must obey the laws of physics
that we have studied in previous chapters. As the Concepts-at-a-Glance chart in
Figure 31-6 reminds us, these laws are called conservation laws because each of
them deals with a property (such as mass/energy, electric charge, linear
momentum, and angular momentum) that is conserved or does not change during a
process. To the first four conservation laws in Figure 31-6, we now add a fifth,
the conservation of nucleon number. In all radioactive decay processes it has
been observed that the number of nucleons (protons plus neutrons) present before
the decay is equal to the number of nucleons after the decay. Therefore, the
number of nucleons is conserved during a nuclear disintegration. As applied to
the disintegration of a nucleus, the conservation laws require that the energy,
electric charge, linear momentum, angular momentum, and nucleon number that a
nucleus possesses must remain unchanged when it disintegrates into nuclear
fragments and accompanying  ,
, 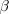 , or
, or  rays.
rays. 
 |
 |
|
|
 |
Figure 31-6 CONCEPTS AT A GLANCE The
conservation laws listed at the left side of this chart are
obeyed when a nucleus undergoes radioactive decay. The three
types of naturally occurring decay are  decay, decay, 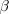 decay, and
decay, and  decay. Nuclear medicine uses
radioactive decay to produce scans of organs. This photograph
shows a nuclear scan of two kidneys, the one on the left
displaying an invasive cancer. (ISM/Phototake) decay. Nuclear medicine uses
radioactive decay to produce scans of organs. This photograph
shows a nuclear scan of two kidneys, the one on the left
displaying an invasive cancer. (ISM/Phototake)
| |
 | |
 |
A magnetic field is directed perpendicular to the plane of the paper, and a
photographic plate is positioned to the right of the hole. Three spots appear on
the developed plate, which are associated with the radioactivity of the nuclei
in the material. Since moving particles are deflected by a magnetic field only
when they are electrically charged, this experiment reveals that two types of
radioactivity (

and
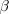
rays, as
it turns out) consist of charged particles, whereas the third type (

rays) does
not.
 |
α Decay |
 |
When a nucleus disintegrates and produces

rays, it is said to undergo
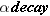
. Experimental
evidence shows that

rays consist of positively charged particles, each
one being the
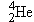
nucleus of helium. Thus, an

particle has a charge of +2
e and a nucleon number of
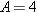
. Since the grouping of 2 protons
and 2 neutrons in a
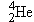
nucleus is particularly stable, as we have seen in
connection with Figure 31-5, it is not surprising that an

particle can be ejected as a unit
from a more massive unstable nucleus.
Figure 31-8 shows the disintegration process for one example of

decay:
The original nucleus is referred to as the
parent nucleus (P), and the nucleus
remaining after disintegration is called the
daughter nucleus (D). Upon emission of an

particle, the
uranium
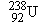
parent
is converted into the

daughter, which is an isotope of thorium. The
parent and daughter nuclei are different, so
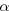
decay converts one element into
another, a process known as
transmutation.
 |
 |
|
|
 |
Figure 31-8  decay occurs when an unstable parent nucleus emits an
decay occurs when an unstable parent nucleus emits an  particle and in the process is converted into a different, or
daughter, nucleus.
particle and in the process is converted into a different, or
daughter, nucleus.
| |
 | |
 |
Electric charge is conserved during

decay. In Figure 31-8, for instance, 90 of the 92
protons in the uranium nucleus end up in the thorium nucleus, and the remaining
2 protons are carried off by the

particle. The total number of 92, however, is the
same before and after disintegration.

decay also conserves the number of nucleons,
because the number is the same before (238) and after (234 + 4) disintegration.
Consistent with the conservation of electric charge and nucleon number, the
general form for

decay is
When a nucleus releases an

particle, the nucleus also releases energy. In
fact, the energy released by radioactive decay is responsible, in part, for
keeping the interior of the earth hot and, in some places, even molten. The
following example shows how the conservation of mass/energy can be used to
determine the amount of energy released in

decay.
 |
Example 4 |
| |
α Decay and the Release of
Energy | |
The atomic mass of uranium 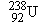 is 238.0508 u, that of thorium  is 234.0436 u, and
that of an  particle 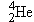 is 4.0026 u. Determine the energy released
when 
decay converts 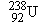 into  .
Reasoning Since energy is
released during the decay, the combined mass of the  daughter nucleus and the  particle is
less than the mass of the 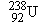 parent nucleus. The difference in mass is
equivalent to the energy released. We will determine the difference in
mass in atomic mass units and then use the fact that 1 u is equivalent to
931.5 MeV.
Solution The decay and the
masses are shown below:
The decrease in mass, or mass defect
for the decay process, is 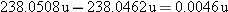 . As usual, the masses are atomic masses and
include the mass of the orbital electrons. But this causes no error here
because the same total number of electrons is included for 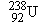 , on the one hand, and
for  plus
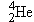 , on the
other. Since 1 u is equivalent to 931.5 MeV, the released energy is 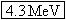 . |
 |
When

decay occurs as in Example 4, the energy released appears as kinetic energy of
the recoiling

nucleus and the

particle, except for a small portion carried away
as a

ray.
Conceptual Example 5 discusses how the

nucleus and the

particle share in the released
energy.
 |
Conceptual Example 5 |
| |
| |
How Energy is Shared During the  Decay of 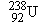
In Example 4, the energy released by the  decay of 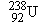 is found to be 4.3 MeV. Since
this energy is carried away as kinetic energy of the recoiling  nucleus and
the 
particle, it follows that 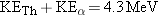 . However, 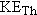 and 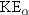 are not equal. Which particle
carries away more kinetic energy, the  nucleus or the  particle?
Reasoning and Solution
Kinetic energy depends on the mass m and speed v of a particle, since 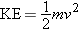 . The  nucleus has a much
greater mass than the  particle, and since the kinetic energy is
proportional to the mass, it is tempting to conclude that the  nucleus has
the greater kinetic energy. This conclusion is not correct, however, since
it does not take into account the fact that the  nucleus and the  particle have
different speeds after the decay. In fact, we expect the thorium nucleus
to recoil with the smaller speed precisely because it has the greater mass. The
decaying 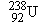
is like a father and his young daughter on ice skates, pushing off against
one another. The more massive father recoils with much less speed than the
daughter. We can use the principle of conservation of linear momentum to
verify our expectation.
As Section 7.2 discusses, the conservation principle states that the
total linear momentum of an isolated system remains constant. An isolated
system is one for which the vector sum of the external forces acting on
the system is zero, and the decaying 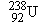 nucleus fits this description. It is
stationary initially, and since momentum is mass times velocity, its
initial momentum is zero. In its final form, the system consists of the
 nucleus
and the 
particle and has a final total momentum of 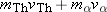 . According to momentum
conservation, the initial and final values of the total momentum of the
system must be the same, so that 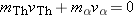 . Solving this equation for the velocity of
the thorium nucleus, we find that 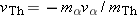 . Since 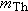 is much greater than 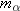 , we can see that the speed of
the thorium nucleus is less than the speed of the  particle. Moreover, the
kinetic energy depends on the square of the speed and only the first power
of the mass. As a result of its much greater speed, the 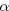 particle has the greater
kinetic energy. particle has the greater
kinetic energy.
Related Homework: Problem 24 |
 |
|
The physics of
radioactivity and smoke
detectors. |
 |
β Decay |
 |
The
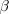
rays
in Figure 31-7 are deflected by the magnetic field in a direction opposite to
that of the positively charged

rays. Consequently, these
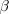
rays, which are the most common
kind, consist of negatively charged particles or

particles. Experiment shows that

particles are
electrons. As an illustration of

decay, consider the thorium

nucleus, which decays by emitting a

particle, as
in Figure 31-10:

decay, like

decay, causes a transmutation of one element into
another. In this case, thorium

is converted into protactinium
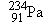
. The law of conservation of charge
is obeyed, since the net number of positive charges is the same before (90) and
after (91 − 1) the

emission. The law of conservation of nucleon number
is obeyed, since the nucleon number remains at
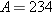
. The general form for

decay is
The electron emitted in

decay does
not actually exist within the parent nucleus
and is
not one of the orbital electrons.
Instead, the electron is created when a neutron decays into a proton and an
electron; when this occurs, the proton number of the parent nucleus increases
from
Z to
Z + 1 and the nucleon number remains
unchanged. The electron is usually fast-moving and escapes from the atom,
leaving behind a positively charged atom.
 |
 |
|
|
 |
Figure 31-10 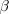 decay occurs when a neutron in an unstable parent nucleus
decays into a proton and an electron, the electron being
emitted as the
decay occurs when a neutron in an unstable parent nucleus
decays into a proton and an electron, the electron being
emitted as the  particle. In the process, the
parent nucleus is transformed into the daughter nucleus. particle. In the process, the
parent nucleus is transformed into the daughter nucleus.
| |
 | |
 |
Example 6 illustrates that energy is released during

decay, just as it is during

decay, and that the
conservation of mass/energy applies.
 |
Example 6 |
| |
| |

Decay and the Release of Energy
The atomic mass of thorium  is 234.043 59 u, and the atomic mass of
protactinium 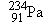 is 234.043 30 u. Find the energy released
when 
decay changes  into 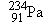 .
Reasoning To find the
energy released, we follow the usual procedure of determining how much the
mass has decreased because of the decay and then calculating the
equivalent energy.
Solution The decay and the
masses are shown below:
When the  nucleus of a thorium atom is
converted into a 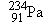 nucleus, the number of orbital electrons
remains the same, so the resulting protactinium atom is missing one
orbital electron. However, the given mass includes all 91 electrons of a
neutral protactinium atom. In effect, then, the value of 234.043 30 u for
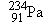 already
includes the mass of the  particle. The mass decrease that accompanies
the 
decay is
The equivalent energy 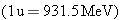 is 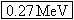 . This is the maximum
kinetic energy that the emitted electron can have.
|
 |
A second kind of
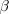
decay sometimes occurs.* In this process the
particle emitted by the nucleus is a
positron rather than an
electron. A positron, also called a
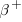
particle, has the same mass as an electron but
carries a charge of +
e instead of −
e. The disintegration process for
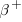
decay is
The emitted positron does
not exist within the nucleus but, rather, is
created when a nuclear proton is transformed into a neutron. In the process, the
proton number of the parent nucleus decreases from
Z to
Z
− 1, and the nucleon number remains the same. As with

decay, the laws of conservation of
charge and nucleon number are obeyed, and there is a transmutation of one
element into another.
 |
γ Decay |
 |
The nucleus, like the orbital electrons, exists only in discrete energy
states or levels. When a nucleus changes from an excited energy state (denoted
by an asterisk *) to a lower energy state, a photon is emitted. The process is
similar to the one discussed in Section 30.3 for the photon emission that leads
to the hydrogen atom line spectrum. With nuclear energy levels, however, the
photon has a much greater energy and is called a

ray. The

decay process is written as
follows:

decay does
not cause a transmutation of one element into
another. In the next example the wavelength of one particular

-ray photon is
determined.
Need more practice?
 |
 |
| Interactive LearningWare31.1 |
Sodium 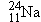 (atomic mass = 23.99 u) emits a  ray
that has an energy of 0.423 MeV. Assuming that the 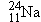 nucleus is
initially at rest, find the speed with which the nucleus recoils.
Ignore relativistic effects.
Related
Homework: Problem
28
| |
|
A N A L Y Z I N G
M U L T I P L E - C O N C E P T
P R O B L E M S |
 |
Example 7 |
| |
The Wavelength of a Photon Emitted During
γ Decay | |
What is the wavelength (in vacuum) of the 0.186-MeV  -ray
photon emitted by radium 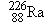 ?
Reasoning The
wavelength of the photon is related to the speed of light and the
frequency of the photon. The frequency is not given, but it can be
obtained from the 0.186-MeV energy of the photon. The photon is
emitted with this energy when the nucleus changes from one energy
state to a lower energy state. The energy is the difference 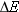 between
the two nuclear energy levels, in a way very similar to that
discussed in Section 30.3 for the energy levels of the electron in
the hydrogen atom. In that section, we saw that the energy
difference 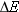 is related to the frequency f and Planck’s constant h, so that we will be able to
obtain the frequency from the given energy value.
Knowns and Unknowns
The following table summarizes the available data:
|
Description |
Symbol |
Value |
Comment |
Energy of  -ray
photon |
|
0.186 MeV |
Will be converted into joules |
|
Unknown
Variable |
|
|
|
Wavelength of  -ray
photon |
|
? |
| |
|
 |
|
Modeling the Problem
|
Step 1 The Relation of Wavelength to
Frequency |
|
The photon wavelength 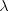 is related to the photon frequency f and the
speed c of light in a vacuum
according to Equation 16.1, as shown at the right. We have no value for
the frequency, so we turn to Step 2 to evaluate it.
|
|
Step 2 Photon Frequency and Photon
Energy |
|
Section 30.3 discusses the fact that the photon emitted when the
electron in a hydrogen atom changes from a higher to a lower energy level
has an energy 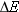 , which is the difference between the energy
levels. A similar situation exists here when the nucleus changes from a
higher to a lower energy level. The  -ray photon that is emitted has an energy 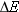 given by 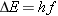 (Equation
30.4). Solving for the frequency, we obtain
which we can substitute into Equation
16.1, as indicated at the right.
Solution Combining the
results of each step algebraically, we find that
The wavelength of the  -ray photon is
Note that we have converted the value
of 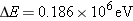 into
joules by using the fact that 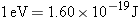 .
Related Homework: Problem
22 |
 |
Medical Applications of Radioactivity |
 |
Gamma Knife radiosurgery is becoming a very promising medical procedure for
treating certain problems of the brain, including benign and cancerous tumors,
as well as blood vessel malformations. The procedure, which involves no knife at
all, uses powerful, highly focused beams of

rays aimed at the tumor or malformation. The

rays are emitted by
a radioactive cobalt-60 source. As Figure 31-11
a illustrates, the patient wears a protective
metal helmet that is perforated with many small holes. Part
b of the figure shows that the holes focus the

rays to a
single tiny target within the brain. The target tissue thus receives a very
intense dose of radiation and is destroyed, while the surrounding healthy tissue
is undamaged. Gamma Knife surgery is a noninvasive, painless, and bloodless
procedure that is often performed under local anesthesia. Hospital stays are 70
to 90% shorter than with conventional surgery, and patients often return to work
within a few days.
 |
 |
|
|
 |
Figure 31-11 (a) In Gamma Knife
radiosurgery, a protective metal helmet containing many small
holes is placed over the patient’s head. (© Custom Medical
Stock Photo) (b) The
holes focus the beams of  rays to a tiny target within the
brain. rays to a tiny target within the
brain.
| |
 | |
 |
|
 The physics
of Gamma Knife
radiosurgery. The physics
of Gamma Knife
radiosurgery.
|
An exercise thallium heart scan is a test that uses radioactive thallium to
produce images of the heart muscle. When combined with an exercise test, such as
walking on a treadmill, the thallium scan helps identify regions of the heart
that do not receive enough blood. The scan is especially useful in diagnosing
the presence of blockages in the coronary arteries, which supply oxygen-rich
blood to the heart muscle. During the test, a small amount of thallium is
injected into a vein while the patient walks on a treadmill. The thallium
attaches to the red blood cells and is carried throughout the body. The thallium
enters the heart muscle by way of the coronary arteries and collects in
heart-muscle cells that come into contact with the blood. The thallium isotope
used,
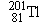
, emits

rays, which a
special camera records. Since the thallium reaches those regions of the heart
that have an adequate blood supply, lesser amounts show up in areas where the
blood flow has been reduced due to arterial blockages (see Figure 31-12). A
second set of images is taken several hours later, while the patient is resting.
These images help differentiate between regions of the heart that temporarily do
not receive enough blood (the blood flow returns to normal after the exercise)
and regions that are permanently damaged due to, for example, a previous heart
attack (the blood flow does not return to normal).
 |
 |
|
|
 |
Figure 31-12 An
exercise thallium heart scan indicates regions of the heart
that receive insufficient blood during exercise.
| |
 | |
 |
|
 The physics
of an exercise thallium heart
scan. The physics
of an exercise thallium heart
scan.
|
 The physics of brachytherapy implants.
The physics of brachytherapy implants. The use of radioactive
isotopes to eliver radiation to specific targets in the body is an important
medical technique. In treating cancer, for example, the method of delivery
should ideally apply a high dose of radiation to a malignant tumor in order to
kill it, while applying only a small (non-damaging) dose to healthy surrounding
tissue. Brachytherapy implants offer such a delivery method. In this type of
treatment radioactive isotopes are formed into small seeds and implanted
directly in the tumor according to a predesigned pattern. The energy and type of
radiation emitted by the isotopes can be exploited to optimize a treatment
design and minimize damage to healthy tissue. Seeds containing iridium
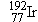
are used to treat
many cancers, and seeds containing iodine
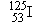
and palladium
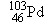
are used for prostate cancer. Research has also
indicated that brachytherapy implants may have an important role to play in the
treatment of atherosclerosis, in which blood vessels become blocked with plaque.
Such blockages are often treated using the technique of balloon angioplasty.
With the aid of a catheter inserted into an occluded coronary artery, a balloon
is inflated to open the artery and place a stent (a metallic mesh that provides
support for the arterial wall) at the site of the blockage. Sometimes the
arterial wall is damaged in this process, and as it heals, the artery often
becomes blocked again. Brachytherapy implants (using iridium
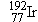
or phosphorus
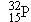
, for instance) have been
found to inhibit repeat blockages following angioplasty.
 Check Your Understanding
3 Check Your Understanding
3 |
Polonium 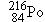 undergoes  decay to produce a daughter
nucleus that itself undergoes  decay. Which one of the following nuclei is
the one that ultimately results: (a) 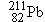 , (b) 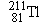 , (c) 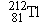 , (d) 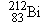 , (e) 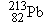 ? (The answer
is given at the end of the book.)
Background: During a
nuclear disintegration, the electric charge and the nucleon number are
conserved, meaning that these quantities remain unchanged when a nucleus
disintegrates into nuclear fragments and the accompanying  and 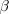 particles.
For similar
questions (including calculational counterparts), consult Self-Assessment
Test 31.1,
which is described at the end of Section
31.5. |
 |
| Copyright © 2007 John Wiley & Sons,
Inc. All rights reserved. |
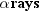 ,
, 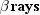 , and
, and 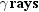 . They are named according to the
first three letters of the Greek alphabet, alpha (
. They are named according to the
first three letters of the Greek alphabet, alpha ( ), beta (
), beta (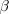 ), and gamma (
), and gamma ( ), to indicate the extent of their
ability to penetrate matter.
), to indicate the extent of their
ability to penetrate matter.  rays are the least penetrating, being blocked by a
thin
rays are the least penetrating, being blocked by a
thin 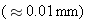 sheet of
lead, whereas
sheet of
lead, whereas 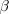 rays penetrate lead to a much greater distance
rays penetrate lead to a much greater distance 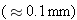 .
.  rays are the most penetrating and
can pass through an appreciable thickness
rays are the most penetrating and
can pass through an appreciable thickness 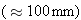 of lead.
of lead. CONCEPTS AT A
GLANCE The nuclear disintegration process that produces
CONCEPTS AT A
GLANCE The nuclear disintegration process that produces  ,
, 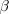 , and
, and  rays must obey the laws of physics
that we have studied in previous chapters. As the Concepts-at-a-Glance chart in
Figure 31-6 reminds us, these laws are called conservation laws because each of
them deals with a property (such as mass/energy, electric charge, linear
momentum, and angular momentum) that is conserved or does not change during a
process. To the first four conservation laws in Figure 31-6, we now add a fifth,
the conservation of nucleon number. In all radioactive decay processes it has
been observed that the number of nucleons (protons plus neutrons) present before
the decay is equal to the number of nucleons after the decay. Therefore, the
number of nucleons is conserved during a nuclear disintegration. As applied to
the disintegration of a nucleus, the conservation laws require that the energy,
electric charge, linear momentum, angular momentum, and nucleon number that a
nucleus possesses must remain unchanged when it disintegrates into nuclear
fragments and accompanying
rays must obey the laws of physics
that we have studied in previous chapters. As the Concepts-at-a-Glance chart in
Figure 31-6 reminds us, these laws are called conservation laws because each of
them deals with a property (such as mass/energy, electric charge, linear
momentum, and angular momentum) that is conserved or does not change during a
process. To the first four conservation laws in Figure 31-6, we now add a fifth,
the conservation of nucleon number. In all radioactive decay processes it has
been observed that the number of nucleons (protons plus neutrons) present before
the decay is equal to the number of nucleons after the decay. Therefore, the
number of nucleons is conserved during a nuclear disintegration. As applied to
the disintegration of a nucleus, the conservation laws require that the energy,
electric charge, linear momentum, angular momentum, and nucleon number that a
nucleus possesses must remain unchanged when it disintegrates into nuclear
fragments and accompanying  ,
, 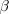 , or
, or  rays.
rays. 
 and
and 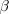 rays, as
it turns out) consist of charged particles, whereas the third type (
rays, as
it turns out) consist of charged particles, whereas the third type ( rays) does
not.
rays) does
not. rays, it is said to undergo
rays, it is said to undergo 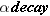 . Experimental
evidence shows that
. Experimental
evidence shows that  rays consist of positively charged particles, each
one being the
rays consist of positively charged particles, each
one being the 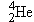 nucleus of helium. Thus, an
nucleus of helium. Thus, an  particle has a charge of +2e and a nucleon number of
particle has a charge of +2e and a nucleon number of 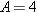 . Since the grouping of 2 protons
and 2 neutrons in a
. Since the grouping of 2 protons
and 2 neutrons in a 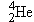 nucleus is particularly stable, as we have seen in
connection with Figure 31-5, it is not surprising that an
nucleus is particularly stable, as we have seen in
connection with Figure 31-5, it is not surprising that an  particle can be ejected as a unit
from a more massive unstable nucleus.
particle can be ejected as a unit
from a more massive unstable nucleus. decay:
decay:
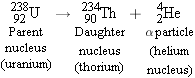
 particle, the
uranium
particle, the
uranium  parent
is converted into the
parent
is converted into the  daughter, which is an isotope of thorium. The
parent and daughter nuclei are different, so
daughter, which is an isotope of thorium. The
parent and daughter nuclei are different, so  decay converts one element into
another, a process known as transmutation.
decay converts one element into
another, a process known as transmutation.
 decay. In Figure 31-8, for instance, 90 of the 92
protons in the uranium nucleus end up in the thorium nucleus, and the remaining
2 protons are carried off by the
decay. In Figure 31-8, for instance, 90 of the 92
protons in the uranium nucleus end up in the thorium nucleus, and the remaining
2 protons are carried off by the  particle. The total number of 92, however, is the
same before and after disintegration.
particle. The total number of 92, however, is the
same before and after disintegration.  decay also conserves the number of nucleons,
because the number is the same before (238) and after (234 + 4) disintegration.
Consistent with the conservation of electric charge and nucleon number, the
general form for
decay also conserves the number of nucleons,
because the number is the same before (238) and after (234 + 4) disintegration.
Consistent with the conservation of electric charge and nucleon number, the
general form for  decay is
decay is
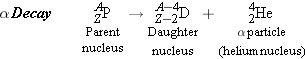
 particle, the nucleus also releases energy. In
fact, the energy released by radioactive decay is responsible, in part, for
keeping the interior of the earth hot and, in some places, even molten. The
following example shows how the conservation of mass/energy can be used to
determine the amount of energy released in
particle, the nucleus also releases energy. In
fact, the energy released by radioactive decay is responsible, in part, for
keeping the interior of the earth hot and, in some places, even molten. The
following example shows how the conservation of mass/energy can be used to
determine the amount of energy released in  decay.
decay.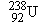 is 238.0508 u, that of thorium
is 238.0508 u, that of thorium  is 234.0436 u, and
that of an
is 234.0436 u, and
that of an  particle
particle 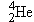 is 4.0026 u. Determine the energy released
when
is 4.0026 u. Determine the energy released
when  decay converts
decay converts 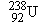 into
into  .
. daughter nucleus and the
daughter nucleus and the  particle is
less than the mass of the
particle is
less than the mass of the 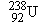 parent nucleus. The difference in mass is
equivalent to the energy released. We will determine the difference in
mass in atomic mass units and then use the fact that 1 u is equivalent to
931.5 MeV.
parent nucleus. The difference in mass is
equivalent to the energy released. We will determine the difference in
mass in atomic mass units and then use the fact that 1 u is equivalent to
931.5 MeV.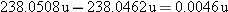 . As usual, the masses are atomic masses and
include the mass of the orbital electrons. But this causes no error here
because the same total number of electrons is included for
. As usual, the masses are atomic masses and
include the mass of the orbital electrons. But this causes no error here
because the same total number of electrons is included for 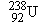 , on the one hand, and
for
, on the one hand, and
for  plus
plus
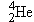 , on the
other. Since 1 u is equivalent to 931.5 MeV, the released energy is
, on the
other. Since 1 u is equivalent to 931.5 MeV, the released energy is 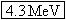 .
.
 decay occurs as in Example 4, the energy released appears as kinetic energy of
the recoiling
decay occurs as in Example 4, the energy released appears as kinetic energy of
the recoiling  nucleus and the
nucleus and the  particle, except for a small portion carried away
as a
particle, except for a small portion carried away
as a  ray.
Conceptual Example 5 discusses how the
ray.
Conceptual Example 5 discusses how the  nucleus and the
nucleus and the  particle share in the released
energy.
particle share in the released
energy. Decay of
Decay of 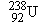
 decay of
decay of 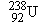 is found to be 4.3 MeV. Since
this energy is carried away as kinetic energy of the recoiling
is found to be 4.3 MeV. Since
this energy is carried away as kinetic energy of the recoiling  nucleus and
the
nucleus and
the  particle, it follows that
particle, it follows that 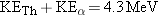 . However,
. However, 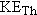 and
and 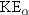 are not equal. Which particle
carries away more kinetic energy, the
are not equal. Which particle
carries away more kinetic energy, the  nucleus or the
nucleus or the  particle?
particle?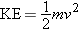 . The
. The  nucleus has a much
greater mass than the
nucleus has a much
greater mass than the  particle, and since the kinetic energy is
proportional to the mass, it is tempting to conclude that the
particle, and since the kinetic energy is
proportional to the mass, it is tempting to conclude that the  nucleus has
the greater kinetic energy. This conclusion is not correct, however, since
it does not take into account the fact that the
nucleus has
the greater kinetic energy. This conclusion is not correct, however, since
it does not take into account the fact that the  nucleus and the
nucleus and the  particle have
different speeds after the decay. In fact, we expect the thorium nucleus
to recoil with the smaller speed precisely because it has the greater mass. The
decaying
particle have
different speeds after the decay. In fact, we expect the thorium nucleus
to recoil with the smaller speed precisely because it has the greater mass. The
decaying 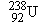 is like a father and his young daughter on ice skates, pushing off against
one another. The more massive father recoils with much less speed than the
daughter. We can use the principle of conservation of linear momentum to
verify our expectation.
is like a father and his young daughter on ice skates, pushing off against
one another. The more massive father recoils with much less speed than the
daughter. We can use the principle of conservation of linear momentum to
verify our expectation.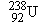 nucleus fits this description. It is
stationary initially, and since momentum is mass times velocity, its
initial momentum is zero. In its final form, the system consists of the
nucleus fits this description. It is
stationary initially, and since momentum is mass times velocity, its
initial momentum is zero. In its final form, the system consists of the
 nucleus
and the
nucleus
and the  particle and has a final total momentum of
particle and has a final total momentum of 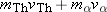 . According to momentum
conservation, the initial and final values of the total momentum of the
system must be the same, so that
. According to momentum
conservation, the initial and final values of the total momentum of the
system must be the same, so that 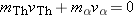 . Solving this equation for the velocity of
the thorium nucleus, we find that
. Solving this equation for the velocity of
the thorium nucleus, we find that 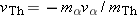 . Since
. Since 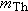 is much greater than
is much greater than 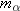 , we can see that the speed of
the thorium nucleus is less than the speed of the
, we can see that the speed of
the thorium nucleus is less than the speed of the  particle. Moreover, the
kinetic energy depends on the square of the speed and only the first power
of the mass. As a result of its much greater speed, the
particle. Moreover, the
kinetic energy depends on the square of the speed and only the first power
of the mass. As a result of its much greater speed, the 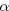 particle has the greater
kinetic energy.
particle has the greater
kinetic energy.
 decay is in smoke detectors. Figure 31-9
illustrates how a smoke detector operates. Two small and parallel metal plates
are separated by a distance of about one centimeter. A tiny amount of
radioactive material at the center of one of the plates emits
decay is in smoke detectors. Figure 31-9
illustrates how a smoke detector operates. Two small and parallel metal plates
are separated by a distance of about one centimeter. A tiny amount of
radioactive material at the center of one of the plates emits  particles, which collide
with air molecules. During the collisions, the air molecules are ionized to form
positive and negative ions. The voltage from a battery causes one plate to be
positive and the other negative, so that each plate attracts ions of opposite
charge. As a result there is a current in the circuit attached to the plates.
The presence of smoke particles between the plates reduces the current, since
the ions that collide with a smoke particle are usually neutralized. The drop in
current that smoke particles cause is used to trigger an alarm.
particles, which collide
with air molecules. During the collisions, the air molecules are ionized to form
positive and negative ions. The voltage from a battery causes one plate to be
positive and the other negative, so that each plate attracts ions of opposite
charge. As a result there is a current in the circuit attached to the plates.
The presence of smoke particles between the plates reduces the current, since
the ions that collide with a smoke particle are usually neutralized. The drop in
current that smoke particles cause is used to trigger an alarm.
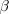 rays
in Figure 31-7 are deflected by the magnetic field in a direction opposite to
that of the positively charged
rays
in Figure 31-7 are deflected by the magnetic field in a direction opposite to
that of the positively charged  rays. Consequently, these
rays. Consequently, these 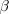 rays, which are the most common
kind, consist of negatively charged particles or
rays, which are the most common
kind, consist of negatively charged particles or  particles. Experiment shows that
particles. Experiment shows that
 particles are
electrons. As an illustration of
particles are
electrons. As an illustration of  decay, consider the thorium
decay, consider the thorium  nucleus, which decays by emitting a
nucleus, which decays by emitting a
 particle, as
in Figure 31-10:
particle, as
in Figure 31-10:
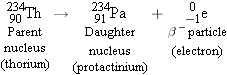
 decay, like
decay, like  decay, causes a transmutation of one element into
another. In this case, thorium
decay, causes a transmutation of one element into
another. In this case, thorium  is converted into protactinium
is converted into protactinium 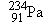 . The law of conservation of charge
is obeyed, since the net number of positive charges is the same before (90) and
after (91 − 1) the
. The law of conservation of charge
is obeyed, since the net number of positive charges is the same before (90) and
after (91 − 1) the  emission. The law of conservation of nucleon number
is obeyed, since the nucleon number remains at
emission. The law of conservation of nucleon number
is obeyed, since the nucleon number remains at 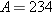 . The general form for
. The general form for  decay is
decay is
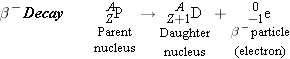
 decay does not actually exist within the parent nucleus
and is not one of the orbital electrons.
Instead, the electron is created when a neutron decays into a proton and an
electron; when this occurs, the proton number of the parent nucleus increases
from Z to Z + 1 and the nucleon number remains
unchanged. The electron is usually fast-moving and escapes from the atom,
leaving behind a positively charged atom.
decay does not actually exist within the parent nucleus
and is not one of the orbital electrons.
Instead, the electron is created when a neutron decays into a proton and an
electron; when this occurs, the proton number of the parent nucleus increases
from Z to Z + 1 and the nucleon number remains
unchanged. The electron is usually fast-moving and escapes from the atom,
leaving behind a positively charged atom.
 decay, just as it is during
decay, just as it is during  decay, and that the
conservation of mass/energy applies.
decay, and that the
conservation of mass/energy applies. Decay and the Release of Energy
Decay and the Release of Energy is 234.043 59 u, and the atomic mass of
protactinium
is 234.043 59 u, and the atomic mass of
protactinium 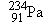 is 234.043 30 u. Find the energy released
when
is 234.043 30 u. Find the energy released
when  decay changes
decay changes  into
into 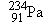 .
. nucleus of a thorium atom is
converted into a
nucleus of a thorium atom is
converted into a 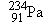 nucleus, the number of orbital electrons
remains the same, so the resulting protactinium atom is missing one
orbital electron. However, the given mass includes all 91 electrons of a
neutral protactinium atom. In effect, then, the value of 234.043 30 u for
nucleus, the number of orbital electrons
remains the same, so the resulting protactinium atom is missing one
orbital electron. However, the given mass includes all 91 electrons of a
neutral protactinium atom. In effect, then, the value of 234.043 30 u for
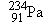 already
includes the mass of the
already
includes the mass of the  particle. The mass decrease that accompanies
the
particle. The mass decrease that accompanies
the  decay is
decay is
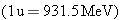 is
is 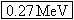 . This is the maximum
kinetic energy that the emitted electron can have.
. This is the maximum
kinetic energy that the emitted electron can have.
 decay sometimes occurs.* In this process the
particle emitted by the nucleus is a positron rather than an
electron. A positron, also called a
decay sometimes occurs.* In this process the
particle emitted by the nucleus is a positron rather than an
electron. A positron, also called a 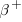 particle, has the same mass as an electron but
carries a charge of +e instead of −e. The disintegration process for
particle, has the same mass as an electron but
carries a charge of +e instead of −e. The disintegration process for 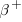 decay is
decay is
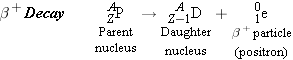
 decay, the laws of conservation of
charge and nucleon number are obeyed, and there is a transmutation of one
element into another.
decay, the laws of conservation of
charge and nucleon number are obeyed, and there is a transmutation of one
element into another. ray. The
ray. The  decay process is written as
follows:
decay process is written as
follows:

 decay does not cause a transmutation of one element into
another. In the next example the wavelength of one particular
decay does not cause a transmutation of one element into
another. In the next example the wavelength of one particular  -ray photon is
determined.
-ray photon is
determined.
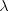 is related to the photon frequency f and the
speed c of light in a vacuum
according to Equation 16.1, as shown at the right. We have no value for
the frequency, so we turn to Step 2 to evaluate it.
is related to the photon frequency f and the
speed c of light in a vacuum
according to Equation 16.1, as shown at the right. We have no value for
the frequency, so we turn to Step 2 to evaluate it.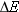 , which is the difference between the energy
levels. A similar situation exists here when the nucleus changes from a
higher to a lower energy level. The
, which is the difference between the energy
levels. A similar situation exists here when the nucleus changes from a
higher to a lower energy level. The  -ray photon that is emitted has an energy
-ray photon that is emitted has an energy 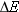 given by
given by 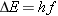 (Equation
30.4). Solving for the frequency, we obtain
(Equation
30.4). Solving for the frequency, we obtain
 -ray photon is
-ray photon is
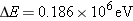 into
joules by using the fact that
into
joules by using the fact that 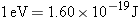 .
. rays aimed at the tumor or malformation. The
rays aimed at the tumor or malformation. The  rays are emitted by
a radioactive cobalt-60 source. As Figure 31-11a illustrates, the patient wears a protective
metal helmet that is perforated with many small holes. Part b of the figure shows that the holes focus the
rays are emitted by
a radioactive cobalt-60 source. As Figure 31-11a illustrates, the patient wears a protective
metal helmet that is perforated with many small holes. Part b of the figure shows that the holes focus the
 rays to a
single tiny target within the brain. The target tissue thus receives a very
intense dose of radiation and is destroyed, while the surrounding healthy tissue
is undamaged. Gamma Knife surgery is a noninvasive, painless, and bloodless
procedure that is often performed under local anesthesia. Hospital stays are 70
to 90% shorter than with conventional surgery, and patients often return to work
within a few days.
rays to a
single tiny target within the brain. The target tissue thus receives a very
intense dose of radiation and is destroyed, while the surrounding healthy tissue
is undamaged. Gamma Knife surgery is a noninvasive, painless, and bloodless
procedure that is often performed under local anesthesia. Hospital stays are 70
to 90% shorter than with conventional surgery, and patients often return to work
within a few days.
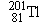 , emits
, emits
 rays, which a
special camera records. Since the thallium reaches those regions of the heart
that have an adequate blood supply, lesser amounts show up in areas where the
blood flow has been reduced due to arterial blockages (see Figure 31-12). A
second set of images is taken several hours later, while the patient is resting.
These images help differentiate between regions of the heart that temporarily do
not receive enough blood (the blood flow returns to normal after the exercise)
and regions that are permanently damaged due to, for example, a previous heart
attack (the blood flow does not return to normal).
rays, which a
special camera records. Since the thallium reaches those regions of the heart
that have an adequate blood supply, lesser amounts show up in areas where the
blood flow has been reduced due to arterial blockages (see Figure 31-12). A
second set of images is taken several hours later, while the patient is resting.
These images help differentiate between regions of the heart that temporarily do
not receive enough blood (the blood flow returns to normal after the exercise)
and regions that are permanently damaged due to, for example, a previous heart
attack (the blood flow does not return to normal).
 The physics of brachytherapy implants. The use of radioactive
isotopes to eliver radiation to specific targets in the body is an important
medical technique. In treating cancer, for example, the method of delivery
should ideally apply a high dose of radiation to a malignant tumor in order to
kill it, while applying only a small (non-damaging) dose to healthy surrounding
tissue. Brachytherapy implants offer such a delivery method. In this type of
treatment radioactive isotopes are formed into small seeds and implanted
directly in the tumor according to a predesigned pattern. The energy and type of
radiation emitted by the isotopes can be exploited to optimize a treatment
design and minimize damage to healthy tissue. Seeds containing iridium
The physics of brachytherapy implants. The use of radioactive
isotopes to eliver radiation to specific targets in the body is an important
medical technique. In treating cancer, for example, the method of delivery
should ideally apply a high dose of radiation to a malignant tumor in order to
kill it, while applying only a small (non-damaging) dose to healthy surrounding
tissue. Brachytherapy implants offer such a delivery method. In this type of
treatment radioactive isotopes are formed into small seeds and implanted
directly in the tumor according to a predesigned pattern. The energy and type of
radiation emitted by the isotopes can be exploited to optimize a treatment
design and minimize damage to healthy tissue. Seeds containing iridium 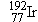 are used to treat
many cancers, and seeds containing iodine
are used to treat
many cancers, and seeds containing iodine 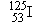 and palladium
and palladium 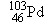 are used for prostate cancer. Research has also
indicated that brachytherapy implants may have an important role to play in the
treatment of atherosclerosis, in which blood vessels become blocked with plaque.
Such blockages are often treated using the technique of balloon angioplasty.
With the aid of a catheter inserted into an occluded coronary artery, a balloon
is inflated to open the artery and place a stent (a metallic mesh that provides
support for the arterial wall) at the site of the blockage. Sometimes the
arterial wall is damaged in this process, and as it heals, the artery often
becomes blocked again. Brachytherapy implants (using iridium
are used for prostate cancer. Research has also
indicated that brachytherapy implants may have an important role to play in the
treatment of atherosclerosis, in which blood vessels become blocked with plaque.
Such blockages are often treated using the technique of balloon angioplasty.
With the aid of a catheter inserted into an occluded coronary artery, a balloon
is inflated to open the artery and place a stent (a metallic mesh that provides
support for the arterial wall) at the site of the blockage. Sometimes the
arterial wall is damaged in this process, and as it heals, the artery often
becomes blocked again. Brachytherapy implants (using iridium 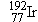 or phosphorus
or phosphorus 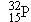 , for instance) have been
found to inhibit repeat blockages following angioplasty.
, for instance) have been
found to inhibit repeat blockages following angioplasty. Check Your Understanding
3
Check Your Understanding
3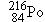 undergoes
undergoes  decay to produce a daughter
nucleus that itself undergoes
decay to produce a daughter
nucleus that itself undergoes  decay. Which one of the following nuclei is
the one that ultimately results: (a)
decay. Which one of the following nuclei is
the one that ultimately results: (a) 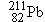 , (b)
, (b) 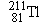 , (c)
, (c) 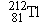 , (d)
, (d) 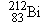 , (e)
, (e) 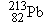 ? (The answer
is given at the end of the book.)
? (The answer
is given at the end of the book.) and
and 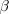 particles.
particles.